The Atom
|
Rest mass: 1.008 - ~300 u Diameter: 50 - 500 x 10 -12 m |
Atoms are the basic modules of matter and the smallest units, in which matter can be fragmented chemically. An atom consists of nucleus and shell. In the nucleus, there are protons and neutrons, it is surrounded by a cloud of electrons. Most of the atom is empty. The number of protons determines, how many electrons it must have to be electrically neutral. Atoms with a different amount of protons and electrons are called ions. The electrons determine the chemical properties of the atoms, so the protons define, of which chemical element the atom is. Atoms with the same amount of protons, but a different amount of neutrons, belong to different isotopes of an element. The number of neutrons is crucial for its stability, that is, if it is more or less radioactive or not at all. Protons and neutrons in the nucleus are kept together by mesons, especially by pions. |
Page last changed on March 06. 2010
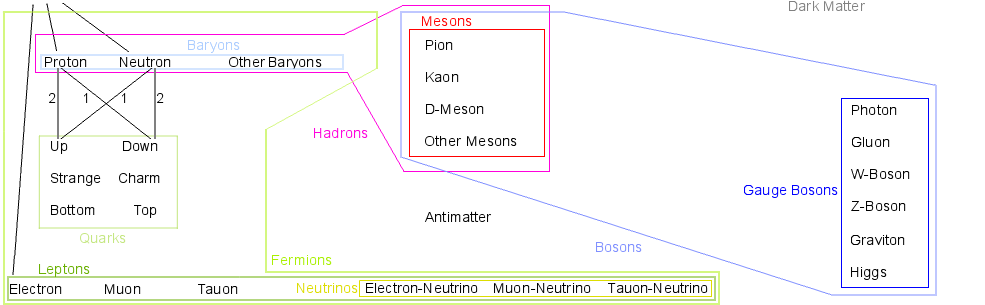
Particle Zoo: Atom, Fermions, Hadrons, Baryons, Proton, Neutron, Other Baryons, Quarks, Up-Quark, Down-Quark, Strange-Quark, Charm-Quark, Bottom-Quark, Top-Quark, Leptons, Electron, Muon, Tauon, Neutrinos, Electron-Neutrino, Muon-Neutrino, Tauon-Neutrino, Bosons, Mesons, Pion, Kaon, D-Meson, Other Mesons, Gauge Bosons, Photon, Gluon, W-Boson, Z-Boson, Graviton, Higgs-Boson, Antimatter, Dark Matter, Dark Energy
↑ Particle Zoo: Atom, Fermions, Hadrons, Baryons, Proton, Neutron, Other Baryons, Quarks, Up-Quark, Down-Quark, Strange-Quark, Charm-Quark, Bottom-Quark, Top-Quark, Leptons, Electron, Muon, Tauon, Neutrinos, Electron-Neutrino, Muon-Neutrino, Tauon-Neutrino, Bosons, Mesons, Pion, Kaon, D-Meson, Other Mesons, Gauge Bosons, Photon, Gluon, W-Boson, Z-Boson, Graviton, Higgs-Boson, Antimatter, Dark Matter, Dark Energy
Advertisement